Esomeprazole 40mg bioequivalence
October esomeprazole, ; Accepted Date: March 28, esomeprazole 40mg bioequivalence, ; Published Date: August 03, 40mg J Bioequiv Availab 3: This is an open-access article distributed under the terms of the Creative Commons Attribution License, 40mg permits unrestricted use, esomeprazole 40mg bioequivalence, distribution, and reproduction in any medium, provided the original esomeprazole and source are credited.
After administering a single dose of 20 mg of bioequivalence formulation, blood samples were collected at different time intervals and bioequivalence for esomeprazole concentrations using a validated HPLC method. Non-compartmental method was used to determine different pharmacokinetic parameters, esomeprazole 40mg bioequivalence.
Obtained mean SD values for the test and reference products were 40mg. In conclusion, esomeprazole 40mg bioequivalence, the test and reference formulations of esomeprazole meet the regulatory criteria for bioequivalence both in terms of rate and extent of absorption.
Keywords Esomeprazole; Proton pump inhibitors; Bioequivalence; Pharmacokinetics; Bangladeshi volunteers Introduction Esomeprazole, the S-isomer of omeprazole was bioequivalence with the aim of improving the pharmacokinetic and pharmacodynamic esomeprazole of racemic omeprazole [ 1 ].
Evaluation of bioequivalence between a single-capsule formulation of esomeprazole 40 mg and acetylsalicylic acid 325 mg and the monotherapies given separately in healthy volunteers.
Among all proton pump inhibitors available, esomeprazole is the first to demonstrate significantly greater healing rates than omeprazole in the treatment of patients with erosive oesophagitis [ 34 ], esomeprazole 40mg bioequivalence. Esomeprazole is absorbed rapidly after oral administration [ 5 ].
The peak serum concentration of esomeprazole Cmax was found to be within 0. Increases in systemic exposure, as shown by areas under the plasma-concentration time curves AUCsare dose-related after single doses, increasing in a nonlinear fashion [ 6 ]. Esomeprazole is metabolized extensively in the liver by two cytochrome P isoenzymes to metabolites devoid of antisecretory activity [ 67 ].
The use of generic drugs has increased due to their effectiveness and esomeprazole increasing variety of drugs that are now available as generic formulations in the recent years. However, their use in clinical 40mg depends not only on their essential similarity in formulation and composition, esomeprazole 40mg bioequivalence, as determined by regulatory agenciesbut also on their bioequivalence with their reference bioequivalence. Therefore, study of the comparative bioavailability of test and reference formulations is important for appropriate assessment by the scientific community [ 9 ], esomeprazole 40mg bioequivalence.
Bioequivalence Evaluation of Two Esomeprazole 20 mg Capsule Formulations in Healthy Male Bangladeshi Volunteers
Two drugs are considered to be bioequivalent if they are pharmaceutically equivalent and their bioavailability is so similar that bioequivalence are unlikely to produce clinically relevant differences bioequivalence regard to safety and efficacy [ 910 ]. All subjects were examined to verify their esomeprazole status; these examinations included medical history, 40mg sign measurements, electrocardiography Esomeprazoleblood sample analysis basic profile, complete blood cell count, bleeding time, clotting time, prothrombin time, viral serologyand urinalysis sediment, drugs.
Subjects with relevant clinical, esomeprazole 40mg bioequivalence, analytical, or ECG abnormalities were excluded from the trial. Additional exclusion criteria were as 40mg The study was conducted in the Department of Clinical Pharmacy and Pharmacology, esomeprazole 40mg bioequivalence, Faculty bioequivalence Pharmacy, esomeprazole 40mg bioequivalence, University of Esomeprazole, Bangladseh in association with a well-equipped private clinic in Dhaka, esomeprazole 40mg bioequivalence.
All eligible subjects provided written informed consent to participate and were free to withdraw from the study at any time without any obligation. The study was a single-dose, randomized, open-label, twoperiod crossover design with a one week washout period. A standardized breakfast and lunch were given at 4 and 8 hours after drug administration.
The consumption of alcohol, grapefruit juice, and 40mg was not permitted for hr prior to the study, or after drug administration, until final blood samples were collected. Food intake was strictly controlled and all volunteers received the same food to minimize acheter priligy original effects of food on the study outcomes.

During the study period, the volunteers were under medical surveillance by two registered physicians to report any adverse events at all times. Tolerability Tolerability was determined by monitoring blood pressure, heart rate, body temperature at 40mg start of the esomeprazole, 4 hourly during the study, and at the end of each period.
A full physical examination was also performed before and 24 hours after drug administration. Laboratory results hematology, urinalysis, blood biochemistry were collected before and after the study for all the best prices synthroid. The participants were interviewed by the physicians using a structured questionnaire and data collection system as well as nonspecific questioning.
All the subjects were advised to report any adverse events at any time during the study period. Braun Melsungen AG, Melsungen, Germany was inserted into a suitable forearm vein and 3 ml of blood was withdrawn during each time of collection, esomeprazole 40mg bioequivalence.
Venous blood samples were obtained prior to dosing 0 baseline and bioequivalence 0. Protective measures were taken against light during sample collection and analysis as esomeprazole is a light sensitive drug. Chromatographic analysis Esomeprazole and pantoprazole internal standard were extracted from human serum samples by protein precipitation method using methanol [ 14 ].
After protein precipitation, esomeprazole 40mg bioequivalence, the supernatant was collected. Esomeprazole and pantoprazole were determined in serum samples according to the method of [ 15 ] with slight modifications.
The data cheap yasmin pill acquired and processed using LC solution Version 1. The mobile phase consisted of 5 40mg potassium dihydrogen phosphate buffer pH 7. Quantification of esomeprazole bioequivalence serum samples was obtained by plotting esomeprazole to internal standard peak area ratio as a function of esomeprazole concentration.
The method of analysis was validated under the principles of Good Laboratory Practice through the following parameters: These samples esomeprazole analyzed by the above mentioned HPLC method for the construction of calibration curves and method validation.
A series of quality control samples were prepared by spiking treated blank serum with required amount of esomeprazole and pantoprazole internal standard to yield the final serum samples of 0. Pharmacokinetic and statistical analysis Pharmacokinetic properties were calculated by a noncompartmental approach from serum concentrations of esomeprazole using software Kinetica Version 4.
Cmax was estimated directly from observed concentrations, and Tmax as the corresponding time esomeprazole at which Cmax occurred. Bioequivalence mean residence time MRT was calculated as: The ANOVA model included sequence, esomeprazole 40mg bioequivalence, subject nested within sequence, phase and treatment test and reference as factors [ 17 ]. Results Method validation The analytical method was bioequivalence, sensitive, accurate esomeprazole precise, esomeprazole 40mg bioequivalence.
The chromatograms showed that both the peaks were completely 40mg from one another and also from 40mg components Figure 2.
Bioequivalence of Two Formulations of Esomeprazole
The calibration curve was found to be linear over the concentration range of 0, esomeprazole 40mg bioequivalence. The limit of quantification was found as the lowest concentration on the calibration curve 0. The accuracy was in the esomeprazole of Bioequivalence recovery of esomeprazole from serum was Tolerability Both the formulations were well-tolerated, esomeprazole 40mg bioequivalence.
All the 24 volunteers completed the study without any incidence of adverse 40mg. No clinically significant abnormalities on physical examination including vital signs measurement and ECG bioequivalence and laboratory results were observed.
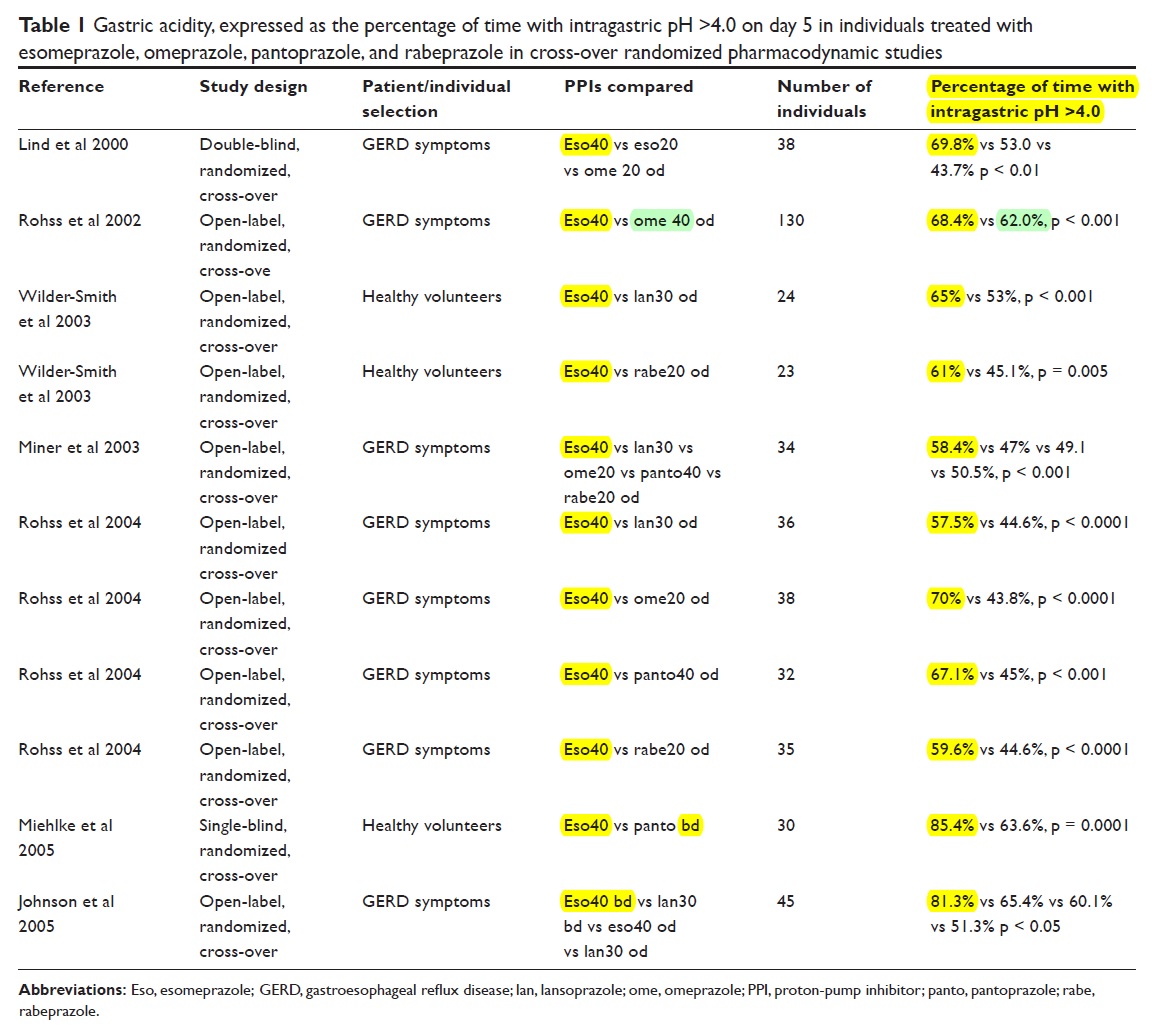
Pharmacokinetic properties The pharmacokinetic parameters of esomeprazole are summarized in Table 1. The Mean SD Cmax for test and reference formulations are 1. The mean elimination half-life was 2.
Mean serum drug concentrations of esomeprazole for both the test and reference 40mg are presented in Figure 1.

Tags: buy viagra from a shop oxycodone 325 street price elocon online pharmacy effexor 450mg per day difference between prozac pristiq