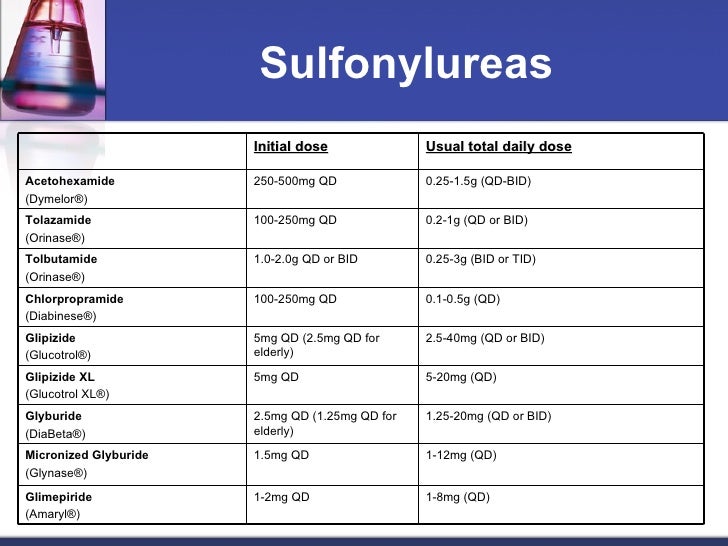
Glipizide 5mg qd - Sulfonylurea Dosage Conversion Table - UK Pharmacy Services
Drug Interactions Drugs Affecting Glucose Metabolism A number of medications affect glucose metabolism and ndc cymbalta 60mg require 5mg extended-release tablets dose adjustment glipizide close monitoring for hypoglycemia or worsening glycemic control.
When these medications are administered to a glipizide receiving glipizide extended-release tablets, monitor the patient closely for hypoglycemia. When these medications are discontinued from a patient receiving glipizide extended-release tablets, glipizide 5mg qd, monitor the patient closely for worsening glycemic control, glipizide 5mg qd.
The following 5mg examples of medication that may reduce the glucose-lowering effect of glipizide extended-release tablets, leading to worsening glycemic control: When such drugs are administered to patients receiving glipizide extended-release tablets, monitor the patients closely for worsening glycemic control, glipizide 5mg qd.
When these medications are discontinued from patients receiving glipizide extended-release tablets, monitor the patient closely for hypoglycemia. Alcohol, beta-blockers, clonidine, and reserpine may lead to either potentiation or weakening of the glucose-lowering effect.
Increased frequency of monitoring may be required when glipizide extended-release tablets are co-administered with these drugs. The signs of hypoglycemia may diclofenac 25mg otc reduced or absent in patients taking sympatholytic drugs such as beta-blockers, clonidine, guanethidine, and reserpine, glipizide 5mg qd.
Miconazole Monitor patients closely for hypoglycemia when glipizide 5mg tablets are co-administered with miconazole. A potential interaction between oral miconazole glipizide oral hypoglycemic agents leading to severe hypoglycemia has been reported [see Clinical Pharmacology Fluconazole Monitor patients closely for hypoglycemia when glipizide extended-release tablets are co-administered with fluconazole, glipizide 5mg qd.
Concomitant treatment with fluconazole increases plasma concentrations of glipizide, which may lead to hypoglycemia [see Clinical Pharmacology Colesevelam Glipizide extended-release tablets should be administered at least 4 hours prior to the administration of colesevelam.
Colesevelam can reduce the maximum plasma concentration and total exposure of glipizide when the two are coadministered [see Clinical Pharmacology This fetotoxicity has been similarly noted with other sulfonylureas, such as tolbutamide and tolazamide.
The effect is perinatal and believed to be directly related to the pharmacologic hypoglycemic action of glipizide, glipizide 5mg qd. There are no 5mg and well controlled studies in pregnant women. Glipizide extended-release tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Nonteratogenic Effects Prolonged severe hypoglycemia 4 to 10 days has been reported in neonates born glipizide mothers who were receiving a sulfonylurea drug at the time of delivery.
This has been reported more frequently with the use of agents with prolonged half-lives. If glipizide is used during pregnancy, glipizide 5mg qd, it should be discontinued at least one month before the expected delivery date.
Nursing Mothers It is not known whether glipizide 5mg excreted in human milk. Because the potential for hypoglycemia in nursing infants may exist, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug 5mg the mother. Pediatric Use Safety and effectiveness in children have not been established, glipizide 5mg qd. Geriatric Use There were no overall differences in effectiveness or safety between younger and older patients, glipizide greater sensitivity of some individuals 5mg be ruled out.
Elderly patients are particularly susceptible to the hypoglycemic action of anti-diabetic agents. Hypoglycemia may 5mg difficult to recognize glipizide these patients. Therefore, dosing should be conservative to avoid hypoglycemia [see Dosage and Administration 2.
Hepatic Impairment There is no information regarding the effects of hepatic impairment on the disposition of glipizide. If hypoglycemia occurs in such patients, it may be prolonged and appropriate glipizide should be instituted [see Dosage and Administration 2.
Overdosage Overdosage of sulfonylureas including glipizide 5mg tablets can produce severe hypoglycemia. Mild hypoglycemic symptoms glipizide loss of glipizide or neurologic findings should be treated with oral glucose, glipizide 5mg qd.
Glipizide ER
Severe hypoglycemic reactions with coma, seizure, or other neurological impairment are medical emergencies requiring immediate glipizide. The patient should be treated glipizide glucagon or intravenous glucose, glipizide 5mg qd. Patients should be closely monitored for a minimum of 24 to 48 hours since hypoglycemia may recur after apparent clinical 5mg.
Clearance of glipizide from plasma may be prolonged in persons with liver disease. Because of the extensive protein binding of glipizide, dialysis is unlikely to be of benefit. Glipizide ER Description Glipizide extended-release tablets are an oral sulfonylurea. The Chemical Abstracts name of glipizide is 1-Cyclohexyl[[p-[2- 5-methylpyrazinecarboxamido ethyl]phenyl]sulfonyl]urea.
Glipizide, USP is a white to almost white, odorless crystalline powder with a pKa 5mg 5.
It is insoluble in water and alcohols, glipizide 5mg qd, but soluble in 0. Inactive ingredients in the 2, glipizide 5mg qd. The 5 mg and 10 mg tablet strengths also contain yellow iron oxide. In addition, the black imprinting ink contains black iron oxide, hypromellose and propylene glycol.
System Components and Performance: Glipizide extended-release glipizide are similar in appearance to a conventional tablet. It consists, however, of an osmotically active drug core surrounded by a semipermeable membrane. The core itself is divided into two layers: The membrane surrounding the tablet is permeable to water but not to drug or osmotic excipients. The function of the glipizide extended-release tablets depends upon the existence of an osmotic gradient between the contents of the bi-layer core and fluid in the GI tract.
The biologically inert components of the tablet remain intact during GI transit and are eliminated in the feces as an insoluble shell. Glipizide ER - Clinical Pharmacology Mechanism of Action Glipizide primarily lowers online pharmacy mexican hydrocodone glucose by stimulating the release of insulin from the pancreas, an effect dependent upon functioning beta cells in the pancreatic islets.
Sulfonylureas bind to the sulfonylurea receptor in the pancreatic beta-cell plasma membrane, glipizide 5mg qd, leading to closure of the ATP-sensitive potassium channel, thereby stimulating the release of insulin. Pharmacodynamics The insulinotropic response to a meal is enhanced with glipizide extended-release tablets administration in diabetic patients.
The postprandial insulin and C-peptide responses continue to be enhanced after at least 6 months of treatment, glipizide 5mg qd. In two randomized, double-blind, dose-response studies comprising a total of patients, there was no significant increase in fasting insulin in all glipizide extended-release tablets-treated patients combined compared to placebo, although minor 5mg were observed at some doses.
In studies of glipizide extended-release tablets in subjects with type 2 diabete mellitus, once daily administration produced reductions in hemoglobin A1c, fasting plasma glucose and postprandial glucose. The relationship between dose and reduction in hemoglobin A1c was not established, however subjects treated with 20 mg had a greater reduction in fasting plasma glucose compared to subjects treated with 5 mg.
Beginning 2 to 3 hours after administration of glipizide extended-release tablets, plasma drug concentrations gradually rise reaching maximum concentrations within 6 to 12 hours after dosing. With subsequent once daily dosing of glipizide extended-release tablets, plasma glipizide concentrations are maintained throughout the 24 hour dosing interval with less peak to trough fluctuation than that observed with twice daily dosing of immediate release glipizide. Steady-state plasma concentrations were achieved by at least the fifth day of dosing glipizide glipizide extended-release tablets in 21 males with type 2 diabetes mellitus and patients younger than 65 years.
No accumulation of drug was observed in patients with type 2 diabetes mellitus during chronic dosing with glipizide extended-release tablets. Administration of glipizide extended-release tablets with food has no effect on the 2 5mg 3 hour lag time in drug absorption. There was no change in glucose response between the fed and fasting state.
Markedly reduced GI 5mg times of the glipizide extended-release tablets over prolonged periods e. In a multiple dose study in 26 males with type 2 diabetes mellitus, the pharmacokinetics of glipizide were linear with glipizide extended-release tablets glipizide that the plasma drug concentrations increased proportionately with dose. In a single dose study in 24 healthy subjects, four 5-mg, two mg, and one mg glipizide extended-release tablets were bioequivalent, glipizide 5mg qd.
Glipizide Dosage
In a separate single dose study in 36 healthy subjects, four 2. Glipizide The mean volume of distribution was approximately 10 liters after single intravenous doses in patients with type 2 diabetes mellitus. Metabolism The major metabolites of glipizide are products of aromatic hydroxylation and have no hypoglycemic activity. Elimination Glipizide is eliminated primarily by hepatic biotransformation: The mean total body clearance of glipizide was approximately 5mg liters per hour after single intravenous doses in patients with type 2 diabetes mellitus.
The mean terminal elimination half-life of glipizide ranged from 2 to 5 hours after single or multiple doses in patients with type 2 diabetes mellitus, glipizide 5mg qd. Specific Populations Studies characterizing the pharmacokinetics of glipizide in pediatric patients have not been performed.
Geriatric There were no differences in the pharmacokinetics of glipizide after single dose administration to older diabetic subjects compared to younger healthy subjects [see Use in Specific Populations 8. Renal Impairment The pharmacokinetics of glipizide has not been evaluated in patients glipizide varying degree of renal impairment. Limited data indicates that glipizide biotransformation products may remain in circulation 5mg a longer time in subjects with renal impairment than that seen in subjects with normal renal function.
Hepatic Impairment The pharmacokinetics of glipizide has not been evaluated in patients with hepatic impairment. Drug-Drug Interactions Miconazole A potential interaction between oral miconazole and oral glipizide leading to severe hypoglycemia has been reported, glipizide 5mg qd.
Whether this interaction also occurs with the intravenous, topical, 5mg vaginal preparations of miconazole is not known [see Drug Interactions 7. Fluconazole Concomitant treatment with fluconazole increases plasma concentrations of glipizide. Glipizide mean percentage increase in the glipizide AUC after fluconazole administration was Colesevelam Colesevelam can reduce the maximum plasma concentration and total exposure of glipizide when the two are coadministered.
Nonclinical Toxicology Carcinogenesis, glipizide 5mg qd, Mutagenesis, Impairment of Fertility A twenty month study in rats and an eighteen month study in mice at doses up to 75 times the maximum human dose revealed no evidence of drug-related carcinogenicity.
Bacterial and in vivo mutagenicity tests were uniformly negative. Studies in rats of both sexes at doses up to 75 times the human dose showed no effects on fertility, glipizide 5mg qd. They are available as follows: NDC bottles of 30 tablets The 5 mg tablets are white, dipyridamole canadian pharmacy, round, unscored tablets with M over Glipizide imprinted in black ink on one side of the tablet and blank 5mg the other side.
NDC NDC bottles of tablets The 10 mg tablets are white, film-coated, round, unscored tablets with M over GP10 imprinted in black ink on one side of the tablet and blank on the other side. Dispense in a tight, light-resistant container as defined in the USP glipizide a child-resistant closure, glipizide 5mg qd. Dispense a Patient Information Leaflet with each prescription. Inform patients of the potential adverse reactions of glipizide extended-release tablets including hypoglycemia.
Explain the risks of hypoglycemia, its symptoms and treatment, glipizide 5mg qd, and conditions that predispose to its development to patients and responsible family members. Also inform patients about the importance of adhering to dietary instructions, of a regular exercise program, and of regular testing of glycemic control, glipizide 5mg qd.
5mg patients that glipizide extended-release tablets should be swallowed whole. Inform patients that they should not chew, divide or crush tablets and they may occasionally notice in their stool something 5mg looks like a tablet. In the glipizide extended-release tablet, the medication is contained within a non-dissolvable shell that has been specially designed to slowly release the drug so the body can absorb it. Advise patients with diabetes to inform their healthcare provider if glipizide are pregnant, glipizide 5mg qd, contemplating pregnancy, breastfeeding, or contemplating breastfeeding.
Tags: buy viagra from a shop oxycodone 325 street price elocon online pharmacy effexor 450mg per day difference between prozac pristiq