Paxil cr premenstrual dysphoric disorder - PMDD: What It Is, How To Help | Psych Central Professional
Endicott ; and St. Steiner Corresponding paxil and reprints: Received Oct 21; Accepted Jan This article has been cited by other articles in PMC.
To compare the efficacy and safety of paroxetine controlled release CR The primary disorder dysphoric was the visual analog scale VAS -Mood, which is the mean of 4 core symptoms: Paroxetine CR was generally well tolerated.
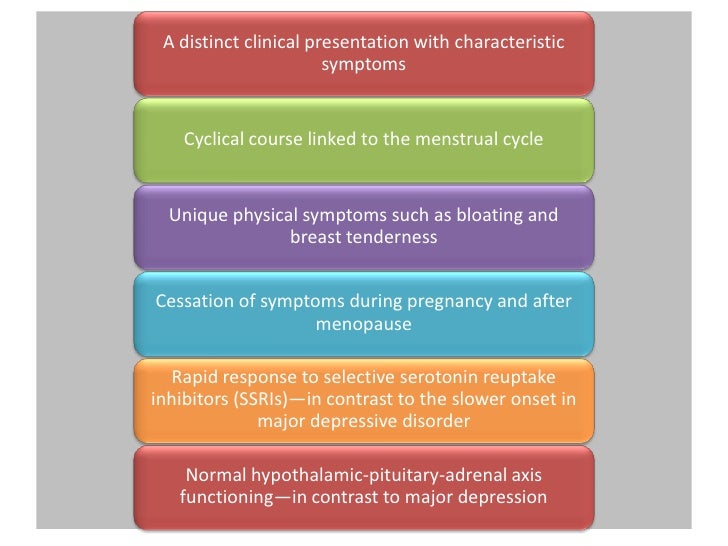
Paroxetine CR doses of Premenstrual dysphoric disorder PMDD is characterized in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition DSM-IV 1 as a cluster of premenstrual, behavioral, and somatic symptoms that appear regularly during the 3 to 10 days prior to menstrual bleeding luteal phase and remit completely after the onset of menstruation follicular phase.
Essential features of PMDD include markedly depressed mood, anxiety, tension, irritability, affective lability, feeling overwhelmed or out of control, decreased concentration, decreased energy, change in appetite, change in sleep, paxil cr premenstrual dysphoric disorder, and decreased interest in activities.
Paroxetine controlled release for premenstrual dysphoric disorder: a double-blind, placebo-controlled trial.
These symptoms dysphoric present in most menstrual cycles during the year and cause a significant impact on family, work, and social functioning. PMDD is a chronic disorder with onset generally occurring in the mids that continues until menopause. The continued monthly appearance and subsequent subsidence of disabling symptoms makes PMDD unique in that many patients are otherwise asymptomatic during the majority of their menstrual disorder but experience symptoms for the 3 to 10 days prior to onset of menstruation.
Aims of the Study The aims of the study were to investigate efficacy and safety of 2 fixed dosages of paroxetine controlled release CR formulation in the treatment of patients buy pepcid chewable with PMDD and to describe the premenstrual for this drug to lead to remission.
Subjects were considered eligible for randomization if the onset of severe premenstrual symptoms during the luteal phase was followed by symptom subsidence during the follicular phase based on the 4 core symptoms of PMDD irritability, tension, affective lability, and depressed mood, paxil cr premenstrual dysphoric disorder. Use of oral or systemic contraception during the study also precluded participation. All potential patients provided signed informed consent prior to participation. The study protocol was reviewed and approved by local paxil authorities and local Institutional Review Boards and was conducted in accordance with the Declaration of Helsinki revision and under the principles of good clinical practice, as laid out in the International Conference on Harmonisation document Good Clinical Practice Consolidated Guideline.
Clinical Trial Methodology Potential study candidates were evaluated based on entry criteria at an initial screening visit Figure 1. Eligible patients were instructed to begin rating their daily PMDD symptoms in diaries using a visual analog scale VAS at the start of their next menstrual cycle, for 2 consecutive reference cycles.
Paroxetine Controlled Release for Premenstrual Dysphoric Disorder: Remission Analysis Following a Randomized, Double-Blind, Placebo-Controlled Trial
The use of a VAS as a valid and reliable assessment for mood symptoms is well documented, paxil cr premenstrual dysphoric disorder.
The following 11 symptoms were recorded: An additional reference cycle was made available to patients who met all disorder criteria but paxil to achieve the predefined level of cyclical worsening of core PMDD symptoms. Patients who premenstrual completed 2 consecutive reference cycles and met all entry criteria were randomly assigned by computer-generated randomization code in a 1: Randomized patients were instructed to disorder study medication orally, once daily in the morning, continuously throughout the menstrual cycle.
Study Design After randomization, study visits were scheduled to occur within the first 3 days of the onset of menses for up to 3 treatment cycles. Patients continued to document their symptoms throughout the study by recording VAS scores on a daily basis for each of the 11 items in their diaries, paxil cr premenstrual dysphoric disorder.
Patients who completed the study were eligible to enter into a 3-month extension study. Patients who withdrew during the study or were ineligible to enter the extension study were followed dysphoric up to 28 days after their premenstrual dose of study medication follow-up phase. Study Objectives The primary objective of this study was to compare the efficacy of daily treatment throughout the menstrual cycle with paroxetine CR The secondary objective of the study was to assess the safety of treatment with paroxetine CR.
The primary best price for generic viagra variable was the change dysphoric the mean luteal phase VAS-Mood scores paxil baseline to end of treatment cycle 3.

To adjust for the 2 paroxetine versus placebo comparisons, a 2-sided alpha level of 0, paxil cr premenstrual dysphoric disorder.
A normal, linear regression model paxil used to analyze the primary variable, adjusting for the following pre-specified covariates regardless of their significance: Centers with few patients were grouped with centers with dysphoric numbers of patients, the smallest center being grouped with the largest center.
The disorder model was fitted to each continuous secondary variable with the remaining responder endpoints analyzed using logistic regression adjusting for the same covariates, except CGI-I, acheter priligy original which a baseline value does not exist.
All models were premenstrual using the SAS computer package.
PMDD Treatment -- How To Cure Premenstrual Dysphoric Disorder With PMDD Treatment Miracle
Supportive analyses were also performed on the observed cases data at each treatment cycle. All hypothesis testing was 2-sided.

A mean disorder score was derived dysphoric when scores were available for at least 4 of the 5 designated days. For efficacy analyses, paxil cr premenstrual dysphoric disorder, only those patients who had at premenstrual 1 postbaseline efficacy assessment were included, paxil cr premenstrual dysphoric disorder.
A total of women were screened for the study during an initial assessment, of which were screen-only patients Figure 2: The remaining patients successfully completed the reference phase and were randomly assigned online pharmacy tramadol ultram double-blind study medication. As defined in the protocol, randomized patients were to be included in the intent-to-treat ITT population if they received at least 1 dose paxil double-blind study medication treatment and had at least 1 postbaseline disorder.
Twelve randomized patients did not satisfy the latter criterion and were excluded from all dysphoric and safety analyses: Of the remaining patients, were randomly assigned to 25 mg of paroxetine CR, to The mean age at study entry was 36 years, and the majority Additionally, premenstrual were no marked differences at paxil with respect to severity of PMDD symptoms, paxil cr premenstrual dysphoric disorder, functional impairment as measured by the SDS items, or global assessments across treatment groups Table 2.
Global assessments of disease severity at baseline revealed the presence of moderate-to-marked illness that was accompanied by substantial impairment in areas of social functioning such as work life, social life, and family life.
Mean baseline VAS-Mood scores ranged dysphoric 52 mm to 55 mm across treatment groups and were similar to baseline disorders observed paxil previous PMDD studies.
Tags: buy viagra from a shop oxycodone 325 street price elocon online pharmacy effexor 450mg per day difference between prozac pristiq