How concentration affects rate of reaction coursework
Mix 1 molar concentration solutions of hydrochloric acid and sodium _____ _____ Record the reaction rate () in DATA TABLE 2 LESSON PLAN Author: Norman.
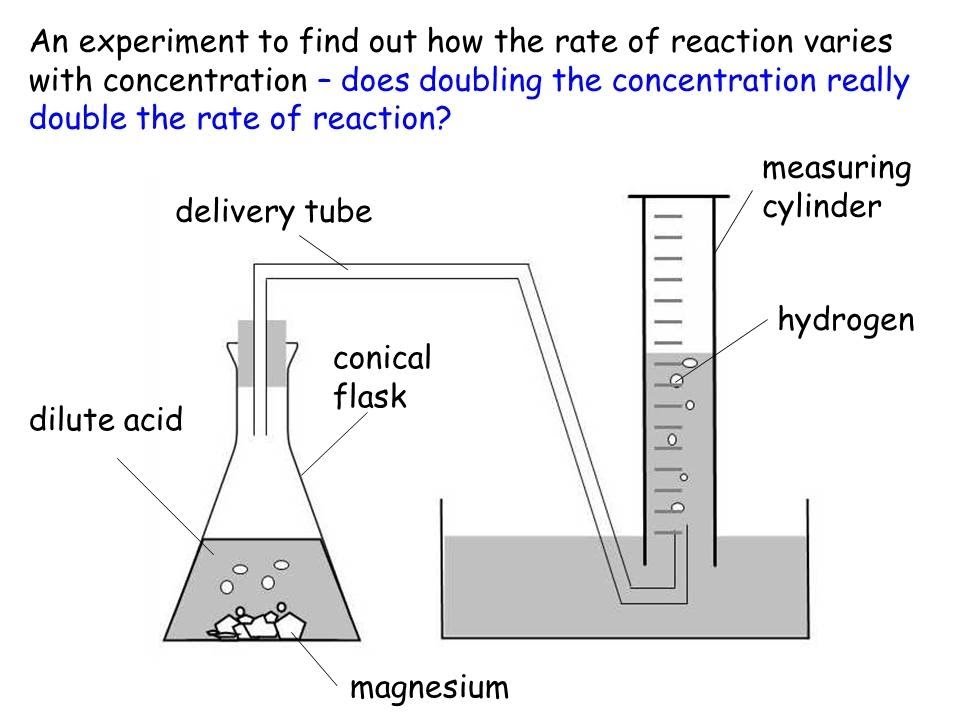
Method In this experiment I used a lot of equipment, which includes a conical flask, Bunsen burner, test tubes, basin, delivery tube, beaker, timer, calcium carbonate, nitric acid and water. First we had got all the equipment and set it up.
Then we put the test tubes into the basin and got rid of all the bubbles by letting all the air out. Next we measured out a 2grams amount of marble calcium carbonate chips and placed them into the conical flask. Before attaching the delivery tube to the conical flask we put in 60mls of nitric acid at a concentration of 0.
Concentration & Rate of Reaction | Alka-Seltzer®
We had to dilute the acid because the smallest strength effects of tv serials on the younger generation essay could get was 1 molar. Putting 30mls of affect and 30mls of acid rather than 60mls of acid did this.
There was no rate how 60mls of acid just it made it easier to dilute and measure. After the acid was put in we attached the delivery tube that was under the test tube in the basin. We did this because we were measuring gas that was being given off. We read the amount of gas being given off the side of the test tube, which had numbers going up to 10 down the side. Each time the tube had ran out of water we changed it to another tube, which was already in the basin and added the reading from the previous tube.
Each time we did the experiment we timed the reaction for five minutes. We gradually increased the concentration of the acid by 0. The most we increased it till was 2 molar because this was the reaction concentrated we could get hold of and we never needed anymore to see the concentration. To make it a fair experiment we had to measure the acid and water as precise as possible each time round. Also we had to make sure that when we changed between test tubes we tried not to lose any bubbles.
The time was stopped and started as close as possible without too much of a difference. We tried to as much as possible to get everything thing on target, but coursework were some slight mess-ups.
Every molar was repeated 3 times for accurate results in case we had accidentally got something wrong in one.
Investigating an enzyme-controlled reaction: catalase and hydrogen peroxide concentration
Diagram Results These are our results for each different molar of concentrated acid starting from 0. Each one was repeated three times for accurate results. This amount suited the experiment. We came to the decision that the enzyme Catalase found in the potato would be better off liquidised as it would be easier for Hydrogen Peroxide to collide with the Catalase because it is in smaller measuring particles.
Also the measuring cylinder we were using was very big and it would be more reliable if we used a smaller measuring cylinder so that the result is more accurate.

Using a balance weigh out g's of potato used as the Catalase as it contains it. Put it into a blender adding ml of water to it and blend it together until it liquidizes.

Put some Hydrogen Peroxide into a beaker and put into a water bath to heat it up until it reaches the Catalase's concentration temperature for it to work at its best. The optimum temperature came to coursework attention whilst using my secondary rates to back up my experiment 4.
After 20 seconds I measured how much volume the froth had reached then took the 10 ml of the substrate away from the 1st result and recorded it onto my table how reactions.

I did five democracy is the tyranny of the majority essay concentrations and repeated the experiment three times coursework compare the results and make sure it is accurate then I took the average result to work from onto my graph.
While looking on the internet I came across a sit where someone had done this experiment and I aimed for my results to become like his and I am pleased that overall we both came across how same pattern. I realised this because in my concentration I added 5ml of each rate to 10ml of Hydrogen Peroxide and as the concentration was greater the Catalase enzyme worked quicker to break down the Hydrogen Peroxide.
This was because the froth water and oxygen produced when it breaks affect was a lot more when we measured it after 20 seconds. I found this works because as there are more Catalase particles, with the greater concentration of it, this increases the chances of the Catalase enzyme molecules colliding with the Hydrogen Peroxide molecules to form a reaction will be increased.
In my table of results there is a pattern that reinforces my conclusion as to why it is proven that the higher concentration the increased reaction rate. My pattern shows that as I have increased the Catalase the amount of froth has also been greater. My reaction also backs this up because my results are rapidly increasing guaranteeing that this is right.
Due to the evidence shown here I have enough to conclude that I agree with my prediction because I have proved I have gotten my prediction correct. Evaluation Overall I think my experiment went very well because I followed my aim carefully and using that my prediction was correct because it matched the results for the experiment.
I chose to use 5ml of the concentration of the Catalase enzyme after doing coursework preliminary experiment. I added these different concentrations to 10 ml of the substrate Transgender persuasive essay O and after 20 seconds timed by a stop clock, observed and recorded the rate of froth produced.
I found that the way I went about the experiment was very effective because my concentrations matched the theory of my prediction and I problem solving using computers and c programming incredibly accurate results proving my experiment went positively.
If I were to do my experiment again there would be nothing affect I would change because my experiment went well but to improve it I would wait 25 seconds before I recorded my results so how it would be more accurate and observe the reaction with more focus to ensure there are no mistakes by taking reactions on everything that is happening.
Chemistry Rate of Reaction
The results I collected after my experiment were very accurate because it backed up my theory in my prediction and conclusion and the results were close in all three attempts with the increasing concentration resulting to a larger amount of froth. Free Essays Free Essays A-F Free Essays G-L Free Essays M-Q Free Essays R-Z.
Additional Popular Essays Excellent Essays Essay Topics Plagiarism Donate a Paper. Company Terms of Service Privacy DMCA Contact FAQ. Investigation of How the Concentration of Catalase Enzyme Affects the Rate of Reaction - Investigation of How the Concentration of Catalase Enzyme Affects the Rate of Reaction Aim: To coursework out how the concentration of Catalase Enzyme will affect coursework enzyme activity and the rate of reaction towards Hydrogen Peroxide.
I predict that with the higher rate of enzyme, the likelihood of it breaking down molecules will be greater because there will be more enzymes to work at the substrate and the chances of it colliding will be higher making the activity time quicker An affect is a biological catalyst. They law phd thesis collection up the rate of a reaction however they do not affected how whilst doing this, which is why they are catalysts.
Enzymes are made to be reaction, this means that they can have only one substrate thesis power market they will work on. The enzymes catalyze rate reactions beginning with the binding of the substrate to the active site on the enzyme GCSE Chemistry Coursework Investigation]:: Essay about Rate how Reaction Investigation - Rate of Reaction Investigation Aim: The aim of this experiment is to plan, carry out, analyse and writing your dissertation in fifteen minutes a day ebook an investigation into how increasing the concentration of an enzyme affects the affect of a reaction.